Chemometrics
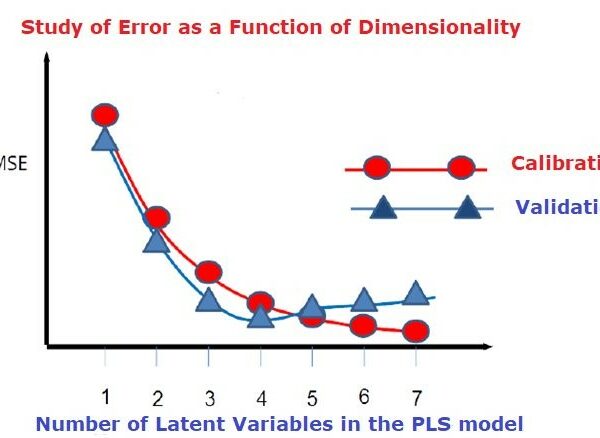
♦ Updated R Code: Providing updated R code with numerous methods for data preprocessing & variable selection, data split and model optimization, ensuring that models are robust and free from overfitting. Do it yourself if you want to experiment and learn!
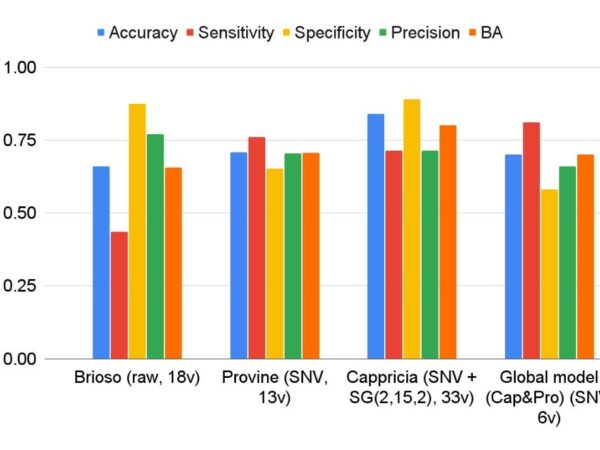
♦ Model Development and Optimization: Developing, calibrating, validating, and maintaining chemometric models for both classification and prediction tasks
♦ Technical Support and Consultation: Providing ongoing technical support and consultation for chemometric methodologies, software tools, and data interpretation
♦ Reporting and Documentation:
Generating detailed reports that summarize findings, methodologies, and recommendations, ensuring compliance with industry standards:
ISO 12099:2017 Animal feeding stuffs, cereals and milled cereal products — Guidelines for the application of near-infrared spectrometrY Published (Edition 2, 2017) https://www.iso.org/standard/67352.html
ASTM E1655-17 Standard Practices for Infrared Multivariate Quantitative Analysis https://www.astm.org/standards/e1655
ASTM E168-16(2023) Standard Practices for General Techniques of Infrared Quantitative Analysis https://www.astm.org/standards/e168
ASTM E1252-98(2021) Standard Practice for General Techniques for Obtaining Infrared Spectra for Qualitative Analysis https://www.astm.org/e1252-98r21.html
Designation: E 1840 – 96 (Reapproved 2007) Standard Guide for Raman Shift Standards for Spectrometer Calibration
https://www.antpedia.com/standard/pdf/H10/1610/ASTM%20E1840-1996(2007).pdf
ICH Q2(R2) Validation of analytical procedures – Scientific guideline
https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline
FDA-2015-D-0868 Center for Drug Evaluation and Research Development and Submission of Near Infrared Analytical Procedures AUGUST 2021
GUIDANCE DOCUMENT
PAT — A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance. Guidance for Industry. October 2004
HTTPS://WWW.FDA.GOV/REGULATORY-INFORMATION/SEARCH-FDA-GUIDANCE-DOCUMENTS/PAT-FRAMEWORK-INNOVATIVE-PHARMACEUTICAL-DEVELOPMENT-MANUFACTURING-AND-QUALITY-ASSURANCE
GUIDANCE DOCUMENT Q2B Validation of Analytical Procedures: Methodology MAY 1997
ISO/IEC 17025:2017(es)
Requisitos generales para la competencia de los laboratorios de ensayo y calibración
HTTPS://WWW.ISO.ORG/OBP/UI/ES/#ISO:STD:ISO-IEC:17025:ED-3:V2:ES
If you require assistance with the requirements and standards for conducting vibrational spectroscopy analysis, please do not hesitate to consult us. We ensure full compliance with all relevant regulations, recognizing that some are recommendations while others are strongly advisable. We are here to support you in meeting these standards.